
16th EBF Open Symposium
Science – Winning the Race
Keeping our finger on the pulse
15-17 November 2023 – Hyatt Regency Tower (Barcelona)
PDF renditions of the PowerPoint presentations can be viewed and downloaded from this page by clicking the specific point on the program. The program itself can be viewed and downloaded by clicking here.
Program @ a glance
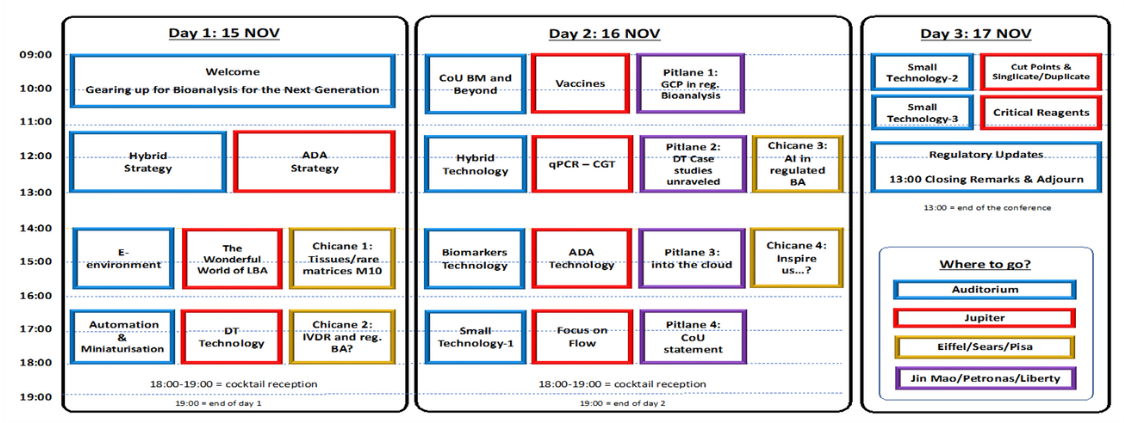
| Day 1 – Wednesday 15 November 2023 | ||
| 09:15 | 09:30 | Welcome |
| 09:30 | 10:30 | Session 1: Gearing up for Bioanalysis for the Next Generation (Plenary) |
| 11:10 | 12:50 | Session 2: Hybrid Assays – Strategy Session 3: ADA – Strategy (Parallel with session 2) |
| 14:00 | 15:40 | Session 4: E-environment Session 5: The Wonderful World of LBA (Parallel with session 4) In the chicane 1: Is everything said on tissues/rare matrices after M10? (Parallel with session 4) |
| 16:20 | 18:00 | Session 6: Automation & Miniaturisation Session 7: Drug Tolerance – Technology (Parallel with session 6) In the chicane 2: IVDR, what is the role for regulated BA? – (Parallel with session 6) |
| Day 2 – Thursday 16 November 2023 | ||
| 09:00 | 10:40 | Session 8: CoU Strategy – Biomarkers and beyond… Session 9: Vaccines (Parallel with session 8) Pitlane 1: GCP (Parallel with session 8) |
| 11:20 | 13:00 | Session 10: Hybrid Assays – Technology and Applications Session 11: qPCR (Parallel with session 10) Pitlane 2: Drug Tolerance case studies unravelled (Parallel with session 10) |
| 14:00 | 15:40 | Session 12: Biomarkers – Technology Session 13: ADA Technology (Parallel with session 12) Pitlane 3: into the cloud (Parallel with session 12) In the chicane 4: Racing for the Future: inspire us….(Parallel with session 12) |
| 16:20 | 18:00 | Session 14: Small molecules – Technology Session 15: Focus on Flow (Parallel with session 14) Pitlane 4: Context of Use (Parallel with session 14) |
| Day 3 – Friday 17 November 2023 | ||
| 09:00 | 10:00 | Session 16: Small Molecules away from Mainstream Session 17: Cut Points & Singlicate/Duplicate (Parallel with session 16) |
| 10:10 | 11:10 | Session 18: Small Molecules – Technology Session 19: Critical Reagents (Parallel with session 18) |
| 11:40 | 13:00 | Session 20: Regulatory Updates (Plenary) |
| 13:00 | 13:15 | Closing remarks – Adjourn |
Details of the sessions
| Day 1 – Wednesday 15 November 2023 | |||
| 09:15 | 09:30 | Welcome | |
| 09:30 | 10:30 | Session 1: Gearing up for Bioanalysis for the Next Generation (Plenary) – Auditorium | |
| In this opening session, we want to take you on board with some of the current and future challenges and opportunities in our industry | |||
| Philip Timmerman, EBF: short introduction to the session | |||
| Matthew Barfield, on behalf of the EBF: AI, a fuel for the future? | |||
| Robert Nelson, on behalf of the EBF: the increasing complexity of clinical trials for BA | |||
| Lauren Stevenson, Immunologix Labs, Rationales, not Rules – Rethinking Guidance for Industry | |||
| 10:30 | 11:10 | Coffee break and Poster Discussion/Viewing | |
| 11:10 | 12:50 | Session 2: Hybrid Assays – Strategy – (Parallel) – Auditorium Session Chair: Matthew Barfield | |
| 11:10 | 11:30 | Matthew Barfield, on behalf of the EBF | |
| Hybrid assays, the scientific and regulatory journey continues | |||
| 11:30 | 11:50 | Alexandra Tavernier, Sanofi | |
| Protein vs peptide immunocapture: the case study of the quantitation of sBCMA | |||
| 11:50 | 12:10 | Nico van de Merbel, University of Groningen | |
| Simultaneous quantification of protein biomarker isoforms by dual immunocapture and LC-MS/MS | |||
| 12:10 | 12:30 | John Perkins, KCAS Bioanalytical & Biomarker Services | |
| Using the Flexibility of Hybrid LC-MS/MS to Address Typical Challenges in Quantitation of Large Molecules | |||
| 12:30 | 12:50 | Shashank Gorityala, BioAgilytix | |
| Strategies and Case Studies on the Bioanalysis of Protein Therapeutics and Biomarkers by Immunoaffinity LC-MS/MS | |||
| 11:10 | 12:50 | Session 3: ADA – Strategy – (Parallel) – Jupiter Session Chair: Jo Goodman, AstraZeneca, on behalf of the EBF | |
| 11:10 | 11:30 | Jo Goodman, on behalf of the EBF | |
| EBF Feedback on Immunogenicity: When to Accelerate and When to Apply the Brakes! | |||
| 11:30 | 11:50 | Daniel Kramer, Sanofi, on behalf of the European Immunogenicity Platform (EIP) | |
| EIP Overview and Cross-Validation of Immunogenicity Assays | |||
| 11:50 | 12:10 | Hanna Widmaier, Nuvisan | |
| Cut-Point Limbo – low cut-points and their challenges | |||
| 12:10 | 12:30 | Claire Seal, invoX Pharma | |
| Case Study of a Neutralising Antibody Assay for FS118, an anti-PD-L1/LAG-3 Bispecific, Tetravalent Antibody | |||
| 12:30 | 12:50 | Nick White, AstraZeneca | |
| Case Study of a Neutralising Antibody Assay for FS118, an anti-PD-L1/LAG-3 Bispecific, Tetravalent Antibody | |||
| 12:50 | 14:00 | Lunch break and Poster Discussion/Viewing | |
| 14:00 | 15:40 | Session 4: E-environment – (Parallel) – Auditorium Session Chair: Cecilia Arfvidsson, AstraZeneca, on behalf of the EBF | |
| 14:00 | 14:20 | Cecilia Arfvidsson, on behalf of the EBF | |
| Moving into the cloud – Are we ready and what actions are needed ? | |||
| 14:20 | 14:40 | Michael Gröschl, CelerionNorbert Bittner, up to data | |
| Introducing Laboratory Automation to regulated bioanalysis: Integration of Liquid Handlers to a paperless laboratory | |||
| 14:40 | 15:00 | Bianca Komoll, LabwareFederico Pastori, Labware | |
| Archiving in GxP Process, Requirements and Pitfalls | |||
| 15:00 | 15:20 | Norbert Bittner, up to data | |
| Case study on the implementation of a regulatory compliant data platform for planning and execution, collaboration, review and reporting of bioanalytical studies. | |||
| 15:20 | 15:40 | Thomas Damberg, Lablytica Life Science | |
| Experiences from 7 years of a paperless laboratory and archiving system under OECD GLP | |||
| 14:00 | 15:40 | Session 5 – The Wonderful World of LBA – (Parallel) – Jupiter Session Chair: Robert Nelson, Bioagilytix, on behalf of the EBF | |
| 14:00 | 14:20 | Roland Staack, Roche Diagnostics (not released for publication) | |
| How to find the “optimal” assay for bioanalytical purposes – can we do better than using the conventional “QC-based” way of assay development and validation? | |||
| 14:20 | 14:40 | Sarah Childs, GSK | |
| Challenges in human tear analysis: Development of a fit-for-purpose qualitative immunoassay to detect biopharmaceutical exposure in rare matrices. | |||
| 14:40 | 15:00 | Yongzhong Zhao, Frontage Laboratories | |
| Mechanistic and Statistic Partitioning the Technical Variability of Ligand Binding Assays in Distinct Formats | |||
| 15:00 | 15:20 | Karien Bloem, Sanquin (not released for publication) | |
| Cross-reactivity of anti-drug antibodies against anti-CD20 therapeutic monoclonal antibodies with other anti-CD20 antibodies | |||
| 15:20 | 15:40 | Panel Discussion | |
| New technologies on the horizon | |||
| 14:00 | 15:40 | In the chicane 1: Is everything said on tissues/secondary matrices after M10? – (Parallel) In Eiffel/Sears/Pisa Session Chair: Steve White, GSK, on behalf of the EBF | |
| In this session, we want to touch base on how the industry looks at tissues and the difference between primary and secondary matrix as defined in the ICH M10 guideline. It builds on prior EBF recommendations and (likely) the outcome of the discussions in the ICH M10 workshop. The theme was chosen because we already observe that industry (and regulators) may not fully use the scientific thinking offered by the ICH M10 and risks at ending up with too tight procedures when tissues/secondary matrices require analysis | |||
| 15:40 | 16:20 | Coffee break and Poster Discussion/Viewing | |
| 16:20 | 18:00 | Session 6: Automation & Miniaturisation – (Parallel) – Auditorium Session Chair: Steve White, GSK, on behalf of the EBF | |
| 16:20 | 16:40 | Mike Wright, GSK | |
| Small steps, Big advances: unleashing the power of miniaturisation and automation for bioanalytical workflows | |||
| 16:40 | 17:00 | David Pekar, Lablytica Life Science | |
| Beginners guide to 384well plate semi-automation for PPT methods using a Liquid Handling Robot | |||
| 17:00 | 17:00 | Laura Boffel, Ghent University (not released for publication) | |
| Near-infrared-based hematocrit prediction using volumetric absorptive microsampling devices: an in-depth evaluation | |||
| 17:20 | 17:40 | James Tunaley, Labcorp Drug Development | |
| Assessment of alternative DNA extraction methods from micro samples of common matrices collected for vector shedding purposes in clinical trials. | |||
| 17:40 | 18:00 | Federico Pastori, ERBC | |
| Hormones Monitoring in Preclinical Development | |||
| 16:20 | 18:00 | Session 7: Drug Tolerance – Technology – (Parallel) – Jupiter Session Chair: Kyra Cowan, Merck KGaA, on behalf of the EBF | |
| 16:20 | 16:40 | Jean-Christophe Genin, F. Hoffmann-La Roche | |
| Let the Biology guide our choices – Case study : Decoding immunogenicity assay performance for reliable ADA data delivery | |||
| 16:40 | 17:00 | Foka Venema, Ardena | |
| Adequate neutralization steps are essential for the development of sensitive, robust and highly drug tolerant anti-drug antibody screening and confirmatory assays | |||
| 17:00 | 17:20 | Gregor Jordan, Roche Diagnostics (not released for publication) | |
| Improving drug tolerance: “An assay perspective” | |||
| 17:20 | 17:40 | Lili Liao, Frontage LaboratoriesAnna Laurén, on behalf of the EBF | |
| Strategies for Improving Drug Tolerance in Immunogenicity Assay | |||
| 17:40 | 18:00 | Brendy Van Butsel (replacing Ortwin Van de Vyver), Sanofi | |
| PandA-monium: are we too tolerant in ADA method development? | |||
| 16:20 | 18:00 | In the chicane 2: IVDR, what is the role for regulated BA? – (Parallel) In Eiffel/Sears/Pisa Session chair: Anna Laurén, NovoNordisk, on behalf of the EBF | |
| In this session, we plan to create an open panel discussion on the challenges and potential mis/over interpretation of the IVDR regulations impacting the regulated BA work. The discussion builds on previous discussions in the EBF, with other partner organisations like the AAPS and work presented at a recent EBF Workshop and AAPS OSD meeting. | |||
| 18:00 | 19:00 | Complementary Cocktail Reception | |
| 19:00 | End of day 1 | ||
| Day 2 – Thursday 16 November 2023 | |||
| 09:00 | 10:40 | Session 8: CoU Strategy – Biomarkers and beyond… – Auditorium Session Chair: Kyra Cowan, Merck KGaA, on behalf of the EBF | |
| 09:00 | 09:20 | Kyra Cowan, on behalf of the EBF | |
| EBF team presentation – BM, qPCR, ADA…CoU is everywhere… | |||
| 09:20 | 09:40 | Nanda Gruben, ICON | |
| Case studies for testing stabilities for biomarker assays | |||
| 09:40 | 10:00 | Heike Wiese, Nuvisan | |
| Metabolomics screening kits for use in clinical trials – fit for purpose? | |||
| 10:00 | 10:20 | Liz Hickford, UCB-Biopharma | |
| A biomarker assay validation approach tailored to the context of use and bioanalytical platform | |||
| 10:20 | 10:40 | Richard Hughes, Resolian | |
| If the shoe doesn’t fit, must we change the shoe? Managing expectations around using ‘off the shelf’ biomarker validations. | |||
| 09:00 | 10:40 | Session 9: Vaccines – (Parallel) – Jupiter Session Chair: Anna Laurén, NovoNordisk, on behalf of the EBF | |
| 09:00 | 09:20 | Stefanie Siegert, AC Immune | |
| Active immunotherapy in neurodegenerative disease: how to define antibody responses to the self-antigen following immunization? | |||
| 09:20 | 09:40 | Floris Loeff, Sanquin | |
| Afucosylated immunoglobulin G responses are a hallmark of enveloped virus infections and are efficiently quantified using the novel fucose-sensitive ELISA for Antigen-Specific IgG (FEASI) assay | |||
| 09:40 | 10:00 | Marijke W.A. Molenaar-de Backer, Sanquin | |
| Hijacking the Monocyte Activation Test from pyrogen test to support immunogenicity testing | |||
| 10:00 | 10:20 | Enric Bertran, Moderna | |
| Bioanalytical challenges for LNP-mRNA vaccines | |||
| 10:20 | 10:40 | Aparna Kasinath, Syngene | |
| Immunogenicity Wanted: Differences between assays for biologics and vaccines | |||
| 09:00 | 10:40 | Pitlane 1: GCP – (Parallel) In Jin Mao/Petronals/Liberty Session Chair: Tsvetelina Ivanova, Comac Medical, on behalf of the EBF | |
| In this workshop, we will share and discuss the challenges related to smart implementation of GCP in the (regulated) BA lab. Focus will be on where and how far we as BA community feel the responsibilities of the BA lab stretches more specifically in relation to the ICF (withdrawal) or when and how is expedited reporting required/mandatory. | |||
| 10:40 | 11:20 | Coffee break and Poster Discussion/Viewing | |
| 11:20 | 13:00 | Session 10: Hybrid Assays – Technology and Applications – (Parallel) – Auditorium Session Chair: Cecilia Arfvidsson, AstraZeneca, on behalf of the EBF | |
| 11:20 | 11:40 | Barry Jones, Crinetics Pharmaceuticals | |
| Quantitation of Adrenocorticotropic Hormone (ACTH) using a Novel Reagent-Free LCMS Assay and Correlation Study to a Clinical Immunoassay | |||
| 11:40 | 12:00 | Abde El Galai, Fox BIOSYSTEMS (not released for publication) | |
| Development of new FO-SPR technology to tackle new challenges with EV and hybrid assay applications. Fox BIOSYSTEMS EIC-project progress | |||
| 12:00 | 12:20 | Michael Blackburn, Quotient Sciences | |
| Hybrid extraction versus physicochemical methods for large peptides: some comparative data and observations | |||
| 12:20 | 12:40 | Linzhi Chen, Boehringer Ingelheim | |
| Development of ELISA plate-based immunocapture for LC/MS/MS analysis of therapeutic proteins | |||
| 12:40 | 13:00 | BRSA Winner 2023: Shivangi Awasthi, Merck & Co., Inc. (known as MSD outside of USA & Canada) | |
| Development of a novel hybrid immunoaffinity-liquid chromatography mass spectrometry (IA-LCMS) approach to supplement ADA testing | |||
| 11:20 | 13:00 | Session 11: qPCR – (Parallel) – Jupiter Session Chair: Anna Laurén, NovoNordisk, on behalf of the EBF | |
| 11:20 | 11:40 | Amanda Hays, on behalf of AAPS | |
| qPCR Support of Cell and Gene Therapies – What to Measure and How | |||
| 11:40 | 12:00 | Lara Duchstein, BioAgilytix | |
| Development and validation of a multiplex qPCR assay for RCL monitoring | |||
| 12:00 | 12:20 | Neil Henderson, AstraZeneca | |
| CRISPR and Applications of Genome Editing: Bioanalytical Strategies & Challenges | |||
| 12:20 | 12:40 | Wibke Lembke, Celerion | |
| AAV8 shedding assay to support gene therapy clinical trials | |||
| 12:40 | 13:00 | Philippe Ancian, Charles River Laboratories | |
| The validation of a duplex qPCR assay to study biodistribution/Shedding of a dual gene therapy vector | |||
| 11:20 | 13:00 | Pitlane 2: Drug Tolerance case studies unravelled (15 min pitch/case studies) – (Parallel) In Jin Mao/Petronals/Liberty Session Chair: Jo Goodman, AstraZeneca on behalf of the EBF | |
| 11:20 | 11:25 | Martin Rieger, MorphoSys AG | |
| Case study: Regulatory interaction with regards to DT on a mAb | |||
| 11:25 | 11:40 | Morten Funch Carlsen, LEO Pharma | |
| Life Cycle Management of ADA and NAb Assays During Clinical Development of a Monoclonal Antibody with Focus on Drug Tolerance Improvement – Nice to Have or Must Have? | |||
| 11:40 | 11:55 | Laura Geary, Resolian | |
| Improving assay performance when complex sample pre-treatment is required – a CRO perspective | |||
| 11:55 | 12:10 | Daniel Dyer, Labcorp Drug Development | |
| Experience of a CRO: Drug Tolerance Case Studies | |||
| 12:10 | 12:25 | Arno Kromminga, BioNTech | |
| ADA Drug Tolerance – Why and when? | |||
| 12:25 | 13:00 | And now…unravel | |
| Panel discussion of the 5 case studies presented | |||
| 11:20 | 13:00 | In the chicane 3: AI in regulated bioanalysis – the future at our doorstep? – (Parallel) In Eiffel/Sears/Pisa Session chair: Matthew Barfield, F. Hoffmann – La Roche, on behalf of the EBF | |
| In this session, we follow up on the presentation from the opening session. AI is likely still far away for many of us, but the EBF feels the this to be the right moment to openly think on the values and risks of AI in support of our work, from early discovery to filing and beyond. The session hopes to surface ‘low hanging’ fruit’ and identify how we should start embracing ‘what is here to stay’. Don’t expect solutions but bring your ideas. | |||
| 13:00 | 14:00 | Lunch break and Poster Discussion/Viewing | |
| 14:00 | 15:40 | Session 12: Biomarkers – Technology – (Parallel) – Auditorium Session Chair: Kyra Cowan, Merck KGaA, on behalf of the EBF | |
| 14:00 | 14:20 | Mouhssin Oufir, KCAS Bio | |
| Challenges of LC-MS/MS method development for the quantitation of a polar low molecular weight biomarker in biological fluids | |||
| 14:20 | 14:40 | Nan Zhang, Frontage Laboratories | |
| Validation of an Ultra-sensitive Method for Phospho-Tau 217 (pTau-217) Quantitation in Human Plasma, Serum and CSF | |||
| 14:40 | 15:00 | Danilo La Terra, Quanterix | |
| Simoa technology enables ultrasensitive biomarker detection | |||
| 15:00 | 15:20 | Alessandro Greco, Aptuit – an Evotec company | |
| Development and validation of a bioanalytical micro LC-MS/MS bottom-up approach method to quantify Semaphorin-3A protein in human plasma samples. | |||
| 15:20 | 15:40 | Panel Discussion | |
| 14:00 | 15:40 | Session 13: ADA Technology – (Parallel) – Jupiter Session Chair: Michaela Golob, Nuvisan, on behalf of the EBF | |
| 14:00 | 14:20 | Valeria Castagna, Merck KGaA | |
| Generic ADA Assay: how to speed up early phase and preclinical immunogenicity testing. | |||
| 14:20 | 14:40 | Sijranke Post, Ardena | |
| The challenges to overcome when developing a synthetic peptide Anti-Drug Antibody assay | |||
| 14:40 | 15:00 | Christopher Tiedje, BioAgilytix | |
| Application of Different Approaches to ADA Domain Specificity Characterization | |||
| 15:00 | 15:20 | Anna Vlachodimou, Genmab (not released for publication) | |
| Novel approach of immunogenicity testing in support of multi-specific antibody drugs | |||
| 15:20 | 15:40 | Anne-Jan Dijkhuis, QPS | |
| Challenges during ADA assay development | |||
| 14:00 | 15:40 | Pitlane 3: into the cloud – (Parallel) In Jin Mao/Petronals/Liberty Session Chair: Cecilia Arfvidsson, AstraZeneca, on behalf of the EBF | |
| In this workshop, we will share and discuss the progress and challenges related to working “in the cloud” in the (regulated) BA. Questions like “are we ready”, “are we aware this is actually happening” or “how to prepare our staff/lab/procedures” to storing or sharing data in the cloud. | |||
| 14:00 | 15:40 | Racing for the Future: inspire us…. In Eiffel/Sears/Pisa | |
| Small discussion booth – bring your ideas to the EBF for future focus We cannot imagine we have been able to touch on all your ideas…tells us our blind spots, so we can consider them for future EBF discussions, where we can be a partner in science or work with the regulators, themes we should prioritise for workshops or symposia…. | |||
| 15:40 | 16:20 | Coffee break and Poster Discussion/Viewing | |
| 16:20 | 18:00 | Session 14: Small molecules – Technology – (Parallel) – Auditorium Session Chair: Steve White, GSK, on behalf of the EBF | |
| 11:00 | 11:20 | Esther van Duijn, TNO | |
| The metabolism of lufotrelvir, a prodrug for the treatment of SARS-COV2, in humans following intravenous administration | |||
| 11:20 | 11:40 | Bertram Nieland, Sciex | |
| Structural elucidation of conjugation drug metabolites by utilizing novel electron-activated dissociation (EAD) | |||
| 11:40 | 12:00 | Ian Wilson, Imperial College London | |
| Doing more with less: Application of microsampling, LC/MS/MS and MS imaging for the measurement of drug, metabolites and lipid biomarkers in biofluids and tissues following the IV & PO administration of gefitinib to the mouse | |||
| 12:00 | 12:20 | Arne Egberts, Merck KGaA | |
| Enhancing Carbohydrate Metabolite and Glycan Analysis through Porous Graphitic Carbon HPLC Columns | |||
| 11:00 | 11:20 | Daniel Schulz-Jander, QPS Netherlands | |
| Oligonucleotide bioanalytical method development – triple quadrupole and high-resolution mass spectrometric detection – the benefits and challenges of selecting the technology. | |||
| 16:20 | 18:00 | Session 15: Focus on Flow – (Parallel) – Jupiter Session Chair: Robert Nelson, BioAgilytix, on behalf of the EBF | |
| 16:20 | 16:40 | Peter van Bommel, ICON | |
| Go with the flow? Ligand binding versus flow cytometry methods for the analysis of anti-drug antibodies in support of CAR-T cell trials | |||
| 16:40 | 17:00 | Petia Doytcheva, Celerion | |
| Sample stability assessments in flow cytometry assays: immunophenotyping case study and critical considerations | |||
| 17:00 | 17:20 | Julian J. Freen-van Heeren, Sanquin (not released for publication) | |
| Considerations for selecting the right flow-based read-out and experimental conditions for development of new antibodies | |||
| 17:20 | 17:40 | Levent Akyüz, CheckImmune | |
| Challenges in Validating Flow Cytometry Panels for Clinical Trials of Cryopreserved Blood Samples | |||
| 17:40 | 18:00 | Johannes Stanta, Celerion | |
| Bringing unstable flow cytometry assays closer to the patient. Case study of an ex-vivo CD11b stimulation flow cytometry assay collected at external clinical site. | |||
| 16:20 | 18:00 | Pitlane 4: Context of Use – (Parallel) In Jin Mao/Petronas/Liberty Session Chair: Kyra Cowan, Merck KGaA, on behalf of the EBF | |
| In this workshop, we will share and discuss the progress and challenges related to implementing the principles of Context to Use for BM assay validation and sample analysis. At the Pitlane-Workshop, which is being prepared by the EBF BM/CoU team, we will engage the audience on the value of a CoU statement as a starting point for CoU discussions between the BA team and the stakeholders/end users of the BM concentration data. | |||
| Case study 1: Richard Hughes – Resolian | |||
| Case study 2: Ulrich Kunz – BI | |||
| Case study 3: Pratiksha Gulati – F. Hoffmann – La Roche | |||
| 18:00 | 19:00 | Complementary Cocktail Reception | |
| 19:00 | End of day 2 | ||
| Day 3 – Friday 17 November 2023 | |||
| 09:00 | 10:00 | Session 16: Small Molecules away from Mainstream – (Parallel) – Auditorium Session Chair: Steve White, GSK, on behalf of the EBF | |
| 09:00 | 09:20 | Tim Vale, Resolian | |
| Road to Recovery: Exploring the challenges in assessing recovery during the validation of an LC-MS method in a rare matrix | |||
| 09:20 | 09:40 | Darren Spark, Charles River Laboratories | |
| Bioanalysis Supporting In-vitro Permeation Tests: Alternatives to Tick-Box Assay Validations | |||
| 09:40 | 10:00 | Nico van de Merbel, Icon | |
| Experiences with development and validation of bioanalytical methods for prodrugs | |||
| 09:00 | 10:00 | Session 17: Cut Points & Singlicate/Duplicate – (Parallel) – Jupiter Session Chair: Michaela Golob, Nuvisan, on behalf of the EBF | |
| 09:00 | 09:20 | Jacomijn Dijksterhuis, ICON | |
| Singlicate analysis applied to pharmacokinetic ligand binding assays: case studies from a CRO perspective | |||
| 09:20 | 09:40 | Issa Jyamubandi, Resolian | |
| A generic singlicate immunogenicity method to detect anti-PEG antibodies: Pre and post dose of pegylated therapies | |||
| 09:40 | 10:00 | James Lawrence, Invox Pharma | |
| It’s all relative, an alternative to the cutpoint approach to Pre-clinical immunogenicity assessment | |||
| 10:00 | 10:10 | Short logistic break | |
| 10:10 | 11:10 | Session 18: Small Molecules – Technology – (Parallel) – Auditorium Session chair: Matthew Barfield, F. Hoffmann – La Roche, on behalf of the EBF | |
| 10:10 | 10:30 | Szabolcs Szarka, Resolian | |
| Design of experiments and automation for the efficient protein LC-MS method development | |||
| 10:30 | 10:50 | Hanna De Baets, Ghent University (not released for publication) | |
| Capillary application of (volumetric) dried blood spot assays for tacrolimus and creatinine determination in stem cell transplant patients | |||
| 10:50 | 11:10 | Alison Dickson, University of St Andrews | |
| Development of a Multi-Modal Imaging Platform for the Analysis of Patient Biopsies in Translational Studies | |||
| 10:10 | 11:10 | Session 19: Critical Reagents – (Parallel) – Jupiter Session Chair: Jo Goodman, AstraZeneca, on behalf of the EBF | |
| 10:10 | 10:30 | Morgan Evans, Agilex Biolabs | |
| Can technology choice make your data SPARCL? | |||
| 10:30 | 10:50 | Annick de Vries, Sanquin | |
| Understanding critical reagent and sample handling in the bioanalytical lab; examples around haemostasis biomarkers | |||
| 10:50 | 11:10 | Paola Genevini, Bio-Rad | |
| Generation of Recombinant Tool Antibodies to Support Cell and Gene Therapy Development | |||
| 11:10 | 11:40 | Coffee Break | |
| 11:40 | 13:00 | Session 20: Regulatory Updates – (Plenary) – Auditorium | |
| 11:40 | 12:30 | Cross validation and ICH M10 – Case studies and Feedback from ICH M10 Workshop | |
| 11:40 – 11:50: Case study 1: Richard Hughes, Resolian | |||
| 11:50 – 12:00: Case study 2: Kamil Sklodowski, F. Hoffmann – La Roche | |||
| 12:00 – 12:10: Case study 3: Daniël Splinter, argenx | |||
| 12:10 – 12:30: Q&A and Feedback from ICH M10 Workshop | |||
| 12:30 | 13:00 | Focus on 3R – Feedback from ICH M10 Workshop (preliminary data – not released for publication) | |
| 12:30 – 12:40: Introduction to surrogate martix experiments for preclinical assays | |||
| 12:50 – 13:00: First results surrogate matrix experiments for preclinical Ligand Binding assays | |||
| 13:00 | 13:15 | Closing remarks – Adjourn – Auditorium | |
| Meeting Organisation: Cecilia Arfvidsson (AstraZeneca), Matthew Barfield (F. Hoffmann – La Roche), Kyra Cowan (Merck KGaA), Michaela Golob (Nuvisan), Jo Goodman (AstraZeneca), Anna Laurén (NovoNordisk), Robert Nelson (BioAgilytix), Steve White (GSK) and Philip Timmerman (EBF) | |||

