
16-18 November 2022 – Barcelona (Spain)
PDF renditions of the PowerPoint presentations can be viewed and downloaded from this page by clicking the specific point on the program. The program itself can be viewed and downloaded by clicking here.
Program @ a glance
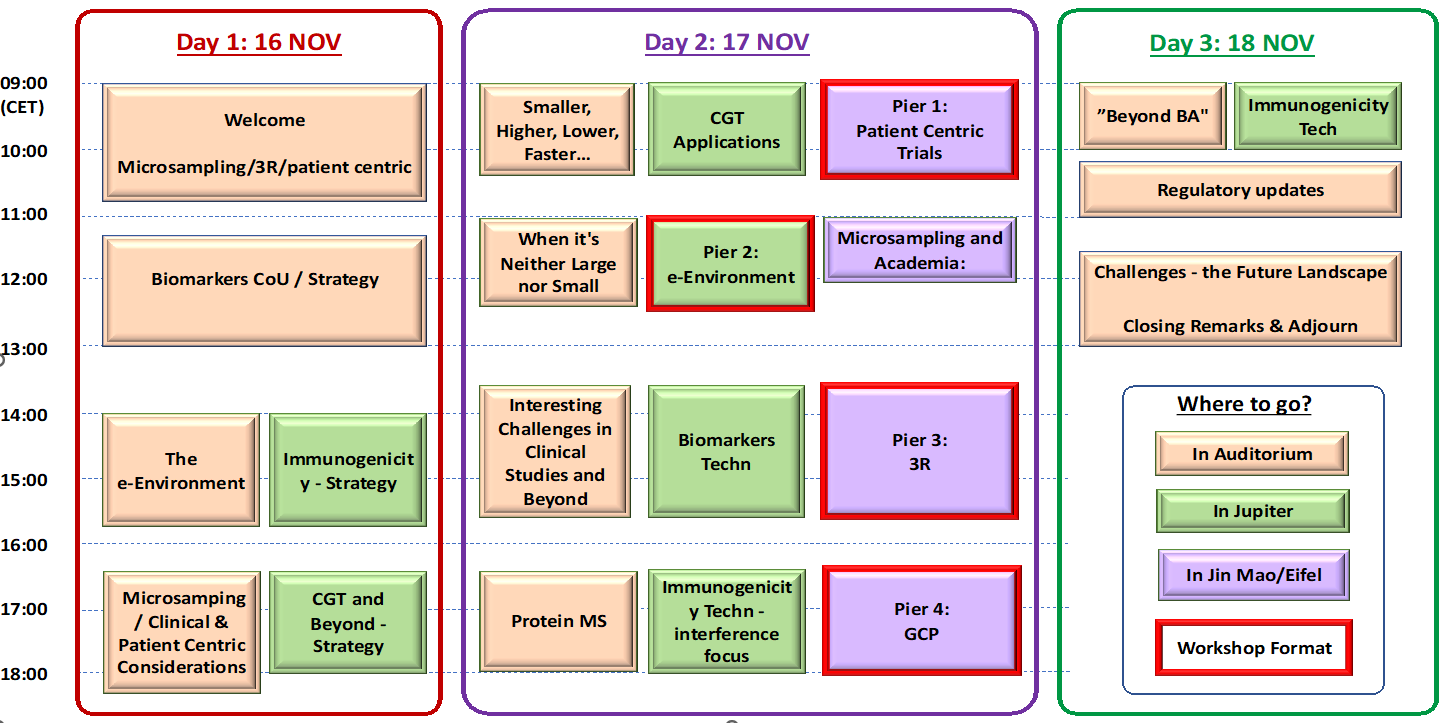
| Day 1 – Wednesday 16 NOV 2022 | ||
| 09:00 | 09:20 | Welcome (Plenary) |
| 09:20 | 10:40 | Starting Big on Small (Plenary) – incl. Introduction to Pier 1-4 Workshops |
| 11:20 | 13:00 | Biomarkers CoU – Strategy |
| 14:00 | 15:40 | The e-Environment parallel with Immunogenicity – Strategy |
| 16:20 | 18:00 | Microsamping / Clinical & Patient Centric Consideration (ends 18:20) parallel with CGT and Beyond – Strategy |
| Day 2 – Thursday 17 NOV 2022 | ||
| 09:00 | 10:20 | Smaller, Lower, Higher, Faster, More, Less….Just Better parallel with CGT – Applications |
| 11:00 | 12:20 | Microsampling and Academia: Where New Ideas are Born parallel with When it’s Neither Large nor Small |
| 14:00 | 16:00 | Interesting Challenges in Clinical Studies and Beyond parallel with Biomarkers Tech |
| 16:40 | 18:00 | Protein MS parallel with Immunogenicity Technology – interference focus |
| 00:00 | Parallel Workshops in the Harbour | |
| 09:00 | 10:20 | Pier 1: Challenges in Patient Centric Trials |
| 11:00 | 12:40 | Pier 2: e-Environment Workshop |
| 14:00 | 16:00 | Pier 3: 3R Workshop |
| 16:40 | 18:00 | Pier 4: GCP Workshop |
| Day 3 – Friday 18 NOV 2022 | ||
| 09:00 | 10:00 | “Beyond BA” parallel with Immunogenicity Tech |
| 10:10 | 11:00 | Regulatory updates (Plenary) |
| 11:00 | 13:00 | Challenges for the Future Landscape (Plenary) |
Details of the sessions
| Day 1 – Wednesday 16 NOV 2022 | ||
| 09:00 | 09:20 | Welcome |
| 09:20 | 10:00 | Starting Big on Small (Plenary) – Auditorium |
| Session chair: Steve White, GSK | ||
| 09:20 | 09:40 | Kevin Bateman, MSD (Merck & Co., Inc.) |
| Patient Centric Sampling and Multi-Omics for Biomarkers in Clinical Development | ||
| 09:40 | 10:00 | Liselotte Björsson, AstraZeneca |
| Microsampling and Singlicate Analysis for Large Molecules delivering Scientific, Ethical and Cost Benefits | ||
| 10:00 | 10:20 | Neil Spooner, Patient Centric Sampling Interest Group and University of Hertfordshire |
| An Overview of Patient Centric Sampling – Overcoming the Hurdles to Implementation | ||
| 10:20 | 10:40 | We meet at the Pier? – Auditorium |
| 00:00 | Short 5-min introduction to the 4 workshops on day 2 by the moderators (all on behalf of the EBF) to introduce the plans and anticipated deliverables | |
| Pier 1: Challenges in Patient Centric Trials – Matthew Barfield | ||
| Pier 2: e-Environment Workshop – Cecilia Arfvidsson | ||
| Pier 3: 3R Workshop – Amanda Wilson (in collaboration with NCR3s) | ||
| Pier 4: GCP Workshop – Tsvetelina Ivanova | ||
| 10:40 | 11:20 | Coffee break |
| 11:20 | 13:00 | Biomarkers CoU – Strategy (Plenary) – Auditorium |
| Session chair: Michaela Golob, Nuvisan | ||
| 11:20 | 11:40 | Nico van de Merbel, ICON plc & U. Groningen |
| Inspiration from another world; validation of LC-MS/MS assays for protein biomarkers in clinical chemistry | ||
| 11:40 | 12:00 | Carmen Fernandez-Metzler, on behalf of the AAPS |
| Does ISR apply for Biomarker Assays? | ||
| 12:00 | 12:20 | Kyra Gelderman, Sanquin Diagnostic Services |
| Complement biomarkers; challenges and solutions around complement measurements. | ||
| 12:20 | 12:40 | Kyra Cowan, on behalf of the EBF |
| Past current and future of EBF discussions, challenges and recommendations on Biomarker/CoU | ||
| 12:40 | 13:00 | Panel Discussion |
| 13:00 | 14:00 | Lunch break |
| 14:00 | 15:40 | The e-Environment (Parallel) – Auditorium |
| Session chair: Cecilia Arfvidsson, AstraZeneca | ||
| 14:00 | 14:20 | Cecilia Arfvidsson, on behalf of the EBF |
| Past, current and future of EBF discussions, challenges and recommendations on secure data transfer | ||
| 14:20 | 14:40 | Norbert Bittner, up to data |
| Applying Software as a Service (SaaS) to Enhance Productivity and Compliance in the End-to-End Workflows of Bioanalytical Labs | ||
| 14:40 | 15:00 | Federico Pastori, Labware |
| A complete Method e-Validation process for all molecules | ||
| 15:00 | 15:40 | Burkhard Schäfer, on behalf of the data transfer vendor consortium |
| The product pathway; a vendor-neutral secure data transfer process between LIMS/ELN and LC-MS/MS instruments for bioanalysis. | ||
| 00:00 | Followed by Panel Discussion on Vendor Neutral Data Transfers – Challenges & Opportunities | |
| 14:00 | 15:40 | Immunogenicity – Strategy (Parallel) – Jupiter |
| Session chair: Robert Nelson, Labcorp Drug Development | ||
| 14:00 | 14:20 | Robert Nelson, on behalf of the EBF |
| Past, current and future of EBF discussions and recommendations on Immunogenicity | ||
| 14:20 | 14:40 | Amanda Hays, on behalf od the AAPS Nab team |
| Neutralizing Anti‐drug Antibody Validation Testing and Reporting Harmonization: Status Update from AAPS | ||
| 14:40 | 15:00 | Dorte Kornerup Ditlevsen, H. Lundbeck A/S |
| A new structured approach to working cross-functionally with immunogenicity | ||
| 15:00 | 15:20 | Jo Goodman, on behalf of the EBF |
| Past, current and future of EBF discussions and recommendations on Cut Points | ||
| 15:20 | 15:40 | Panel Discussion |
| 15:40 | 16:20 | Coffee break |
| 16:20 | 18:20 | Microsamping / Clinical & Patient Centric Considerations (Parallel) – Auditorium |
| Session chair: Matthew Barfield, F. Hoffmann La Roche | ||
| 16:20 | 16:40 | Ryan Lutz, MSD (Merck & Co., Inc.) |
| Evaluation of a Novel Microsampling Device – Tasso-M20 in Support of Clinical PK Studies | ||
| 16:40 | 17:00 | Sofiya Matviykiv, Novartis (Not released for publication) |
| Evaluation of a Tasso blood microsampling device for clinical trial sample collection and biomarker analysis | ||
| 17:00 | 17:20 | Heike Wiese, Nuvisan |
| Comparability of PK data obtained from plasma and whole blood collected with VAMS devices | ||
| 17:20 | 17:40 | Hans Stieltjes, Janssen R&D |
| Practical and Logistical Challenges for Bioanalysis with Dried Patient Centric Sampling devices | ||
| 17:40 | 18:00 | Maurice Steenhuis, Sanquin Diagnostic services |
| Towards the use of fingerprick blood sampling for therapeutic drug monitoring | ||
| 18:00 | 18:20 | Richard Hughes, Drug Development Solutions – Part of Alliance Pharma |
| Micro-managing: exploring the potential to use microsampling technology for quantitation of large molecules | ||
| 16:20 | 18:00 | CGT and Beyond – Strategy (Parallel) – Jupiter |
| Session chair: Anna Laurén, Novo Nordisk | ||
| 16:20 | 16:40 | Johannes Stanta, on behalf of the EBF CGT team |
| Past, current and future of EBF discussions, challenges related to CGT | ||
| 16:40 | 17:00 | Amanda Hays, on behalf of the AAPS qPCR team |
| qPCR in Regulated Bioanalysis- current discussions in the industry | ||
| 17:00 | 17:20 | Teona Roschupkina, Drug Development Solutions – Part of Alliance Pharma |
| Gate that cell: Requirement for analysing Flow Cytometry Data reflection on H62 document | ||
| 17:20 | 17:40 | Anna Laurén, on behalf of the EBF |
| Applying context of use to qPCR method validation and analysis – a progress update. | ||
| 17:40 | 18:00 | Panel Discussion |
| Day 2 – Thursday 17 NOV 2022 | ||
| 09:00 | 10:00 | Smaller, Lower, Higher, Faster, More, Less….Just Better (Parallel) – Auditorium |
| Session chair: Tsvetelina Ivanova, Comac Medical | ||
| 09:00 | 09:20 | Egidijus Machtejevas, Merck KGaA |
| Analysis of Biomacromolecules by LC-MS Utilizing Novel, Narrow-bore, Wide Pore Monolithic and Superficially Porous Stationary Phases | ||
| 09:20 | 09:40 | Cathy Lane, Sciex |
| Achieve sensitive quantification of complex peptides with disulfide bridging in rat plasma using a high-end triple quadrupole mass spectrometer | ||
| 09:40 | 10:00 | George Walters, Drug Development Solutions – Part of Alliance Pharma |
| Exploring the Benefits and Challenges of Universal Automated Methods. | ||
| 09:00 | 10:20 | CGT – Applications (Parallel) – Jupiter |
| Session chair: Anna Laurén, Novo Nordisk | ||
| 09:00 | 09:20 | Michael Schwenkert, SVAR |
| Development of an iLite® Reporter Cell Platform for the Quantification of Anti-AAV Neutralizing Antibodies. | ||
| 09:20 | 09:40 | Lydia Michaut, Tataa |
| Beyond genetic medicine quantification in plasma: validating PCR assays for biodistribution and pharmacodynamic biomarkers | ||
| 09:40 | 10:00 | Zhe Liu, Labcorp Drug Development |
| Detection of Antibodies to AAVs using Gyrolab | ||
| 10:00 | 10:20 | Axel Meyer, Abbvie |
| From quantitative to digital: Validation of a droplet digital (dd)PCR assay | ||
| 09:00 | 10:20 | Pier 1: Workshop on Challenges in Patient Centric Trials (Parallel) – Eiffel/Jin Mao |
| 00:00 | Workshop moderator: Matthew Barfield | |
| Short presentations and discussions to discuss the ‘non-lab’ related issues, i.e. focus on logistic challenges and opportunities for patient centric sampling/trials | ||
| 10:20 | 11:00 | Coffee break |
| 11:00 | 12:20 | Microsampling and Academia: Where New Ideas are Born (Parallel). – Eiffel/Jin Mao |
| Session chair: Steve White, GSK | ||
| 11:00 | 11:20 | Dries Vloemans, KU Leuven |
| Novel self-powered microfluidic platform for advanced remote microsampling applications | ||
| 11:20 | 11:40 | Michele Protti, Unibo |
| Exploring the cannabinoid space with second-generation blood microsampling | ||
| 11:40 | 12:00 | Laura Boffel, Ghent University |
| In-depth evaluation of automated non-contact reflectance-based hematocrit prediction of dried blood spots | ||
| 12:00 | 12:20 | Open Forum: Microsampling in acadamia supporting technology development and new applications |
| 11:00 | 12:20 | When it’s Neither Large nor Small (Parallel) – Auditorium |
| Session chair: Anna Laurén, Novo Nordisk | ||
| 11:00 | 11:20 | Mohammed Abrar, BioApp Solutions Limited (Not released for publication) |
| Bioanalysis of Inbetweeners- (Challenges & Solutions) | ||
| 11:20 | 11:40 | Frida Löthberg, Gyros |
| Strategies for measuring oligonucleotides using a fully automated micro-fluidic immunoassay system | ||
| 11:40 | 12:00 | Michael Blackburn, Quotient Sciences |
| Changing to a Physicochemical Format from Hybrid for a Large Peptide Assay: Pros and Cons | ||
| 12:00 | 12:20 | John Perkins, KCAS Bioanalytical & Biomarker Services |
| Addressing the Impact of Structure on Bioanalysis of Polypeptides & Oligonucleotides | ||
| 11:00 | 12:20 | Pier 2: e-Environment Workshop (Parallel) – Jupiter |
| Workshop moderator: Cecilia Arfvidsson | ||
| Hosted by the EBF e-environment team. During the workshop, the discussion will focus on solutions for secure data transfer for the LBA toolbox and cloud based approaches for the (regulated) BA lab. | ||
| 12:20 | 13:30 | Lunch break |
| 13:30 | 15:30 | Interesting challenges in clinical studies and beyond (Parallel) – Auditorium |
| Session chair: Robert Nelson, Labcorp Drug Development | ||
| 13:30 | 13:50 | Roland Staack, Roche Diagnostics (Not released for publication) |
| Evaluation of free drug/target concentrations by bioanalyis or M&S – do we apply double standards? | ||
| 13:50 | 14:10 | John Chappell, Gyros |
| Development of an assay to measure free IgE as a solution for PK/PD assessment of Omalizumab | ||
| 14:10 | 14:30 | Gareth Whitaker, Quotient Sciences |
| Interim PK analysis and decision making in Translational Pharmaceutics programs | ||
| 14:30 | 14:50 | Aparna Kasinath, Syngene International |
| A simple complex: GLP/GCLP PK, PD and Immunogenicity analysis for GCP clinical studies | ||
| 14:50 | 15:10 | Floris Loeff, Sanquin Diagnostic Services |
| TDM of biologics reassures clinicians in personalised dosing | ||
| 15:10 | 15:30 | Bioanalysis Zone BRSA Winner 2022: Shelby Barnett, Newcastle University (Newcastle, UK) |
| Development of a national therapeutic drug monitoring programme in childhood cancer in the UK | ||
| 13:30 | 15:30 | Biomarkers technical (Parallel) – Jupiter |
| Session chair: Kyra Cowan, Merck KGaA | ||
| 13:30 | 13:50 | Ulrich Kunz, Boehringer Ingelheim |
| Equilibrium (MSD) versus kinetic (Gyrolab) immunoassay in the quantification of a free soluble target – it makes a difference. | ||
| 13:50 | 14:10 | Katja Zeiser, Nuvisan |
| Diving deeper into data: investigation of a CV% issue during a biomarker study using Gyrolab | ||
| 14:10 | 14:30 | Elena Vicentini, Aptuit (Verona) Srl, an Evotec Company |
| High-sensitivity immunoassays for biomarkers of Huntington’s disease | ||
| 14:30 | 14:50 | Yetrib Hathout, Binghamton University |
| Blood accessible biomarkers for Duchenne muscular dystrophy. | ||
| 14:50 | 15:10 | Wikke Berg-Koopmans, ICON plc |
| Challenges and opportunities for determining the dynamics of an unstable cell-membrane marker on a rare cell population by flow cytometry. | ||
| 15:10 | 15:30 | Peter Blattmann, Idorsia Pharmaceuticals |
| Biologically active CXCL12α plasma concentrations increase after multiple-dose treatment with an ACKR3 antagonist in humans | ||
| 13:30 | 15:30 | Pier 3: 3R Workshop (Parallel) – Eiffel/Jin Mao |
| Workshop moderator: Amanda Wilson | ||
| The discussions will focus on identifying opportunities and solutions to minimise usage of experimental animals for in vivo tox/PK studies and how the BA community can reduce usage of blank (rodent) matrices after ICH M10 implementation. | ||
| 15:30 | 16:20 | Coffee Break |
| 16:20 | 18:00 | Protein MS (Parallel) – Auditorium |
| Session chair: Matthew Barfield, F. Hoffmann La Roche | ||
| 16:20 | 16:40 | Matthew Barfield, on behalf of the EBF |
| Past, current and future of EBF discussions, challenges and recommendations on Protein MS | ||
| 16:40 | 17:00 | Szabolcs Szarka, Drug Development solutions |
| Design of Experiment – a Powerful Tool to Optimise Sample Preparation in Bottom-up Targeted Protein LC-MS Workflows | ||
| 17:00 | 17:20 | Ilse De Salve, Merck KGaA |
| Combined affinity capture LC-MS/MS method, for total antibody and conjugated payload quantitation from in-vivo and in-vitro ADC samples. | ||
| 17:20 | 17:40 | Lieve Dillen, Janssen R&D (Not released for publication) |
| Calibration of clinical ELISAs to evaluate immune response by quantitative MS | ||
| 17:40 | 18:00 | Ana Villar Garea, Sanofi (Not released for publication) |
| Comparison of ligand-binding assays and hybrid LC-MS (intact protein) bioanalytical strategies for small therapeutic proteins | ||
| 16:20 | 18:00 | Immunogenicity Technology – Interference Focus (Parallel) – Jupiter |
| Session chair: Jo Goodman, AstraZeneca | ||
| 16:20 | 16:40 | Nick White, AstraZeneca |
| An Old Dog with New Tricks? Resurrection, Reoptimisation and Refinement to Render Drug and Target Interference Redundant | ||
| 16:40 | 17:00 | Presentation cancelled by author |
| cancelled | ||
| 17:00 | 17:20 | Laura Geary, Drug Development Solutions – Part of Alliance Pharma |
| Pushing the Limits of Immunogenicity Assay Drug Tolerance | ||
| 17:20 | 17:40 | Karien Bloem, Sanquin Diagnostic Services |
| Anti-drug antibody testing of therapeutic monoclonal antibodies, have we gone too far? | ||
| 17:40 | 18:00 | Panel Discussion |
| 16:20 | 18:00 | Pier 4: GCP Workshop (Parallel) – Eiffel/Jin Mao |
| Workshop moderator: Tsvetelina Ivanova | ||
| Hosted by the EBF GCP team. During the workshop, we plan to focus on the learnings and Outcome of the GCP Focus Workshop (15-16 September 2022, Malaga) and design/refine recommendations on how to implement GCP requirements into the BA workflows. | ||
| Day 3 – Friday 18 NOV 2022 | ||
| 09:00 | 10:00 | “Beyond BA” (Parallel) – Auditorium |
| Session chair: Tsvetelina Ivanova, Comac Medical | ||
| 09:00 | 09:20 | Claire Szuster, Drug Development Solutions – Part of Alliance Pharma |
| Plasma Protein Binding: On RED alert! | ||
| 09:20 | 09:40 | Humaira Naseer, AstraZeneca (Not released for publication) |
| Overcoming the bioanalytical complexities of nucleotide based biotherapeutics and antibody drug conjugates in tissues derived from non-clinical PK/PD, biodistribution and safety studies | ||
| 09:40 | 10:00 | Gregor Jordan, Roche Diagnostics (Not released for publication) |
| How can the BA expert assist in improving data interpretation and contribute holistically to project support? | ||
| 09:00 | 10:00 | Immunogenicity Tech (Parallel) – Jupiter |
| Session chair: Jo Goodman, AstraZeneca | ||
| 09:00 | 09:20 | Sara Ongay, HEXAL AG (Sandoz a Novartis division) (Not released for publication) |
| Insights into some of the method capabilities and bottlenecks of hybrid LBA-LC-MS/MS for ADA analysis: a case study for a monoclonal antibody (IgG1) | ||
| 09:20 | 09:40 | Phillip Bartlett, Crescendo Biologics (Not released for publication) |
| Detection of Anti-Ig light chains as an immunogenicity assay strategy for novel therapeutic proteins. | ||
| 09:40 | 10:00 | Elisa Bertotti, Merck KGaA |
| Pre-Existing Anti-Drug Antibody evaluation: a standard workflow to support drug candidates selection | ||
| 10:00 | 10:10 | Logistic Break |
| 10:10 | 11:00 | Regulatory updates (Plenary) – Auditorium |
| During the session, we will give an update on recent/upcoming regulatory requirements or challenges for the BA community. The session will build on a pre-meeting survey and ad hoc Q&A. | ||
| 11:00 | 11:30 | Coffee Break |
| 11:30 | 13:00 | Challenges for the Future Landscape (Plenary) – Auditorium |
| Session chair: Kyra Cowan, Merck KGaA | ||
| 11:30 | 11:50 | Foka Venema, Ardena |
| The impact of COVID-19 on BA: accelerating assay development without compromising quality | ||
| 11:50 | 12:10 | Radoiane Helbaj, F. Hoffmann La Roche |
| Biosample Operation Specialist and Bioanalytical manager combined role | ||
| 12:10 | 12:30 | Mari Enoksson, NovoNordisk |
| Challenges and Opportunities for future BA scientists | ||
| 12:30 | 12:45 | Matthew Barfield, on behalf of the EBF |
| View on the changing world and future challenges for the bioanalytical community | ||
| 12:45 | 13:00 | Philip Timmerman, EBF |
| The YSS fueling our future | ||
| 13:00 | Closing Remarks & Adjourn | |
| Meeting organisation: Cecilia Arfvidsson (AstraZeneca), Matthew Barfield (F. Hoffmann – La Roche), Kyra Cowan (Merck KGaA), Michaela Golob (Nuvisan), Jo Goodman (AstraZeneca),Tsvetelina Ivanova (Comac-Medical), Anna Laurén (NovoNordisk), Robert Nelson (Labcorp), Steve White (GSK) and Philip Timmerman (EBF) | ||

